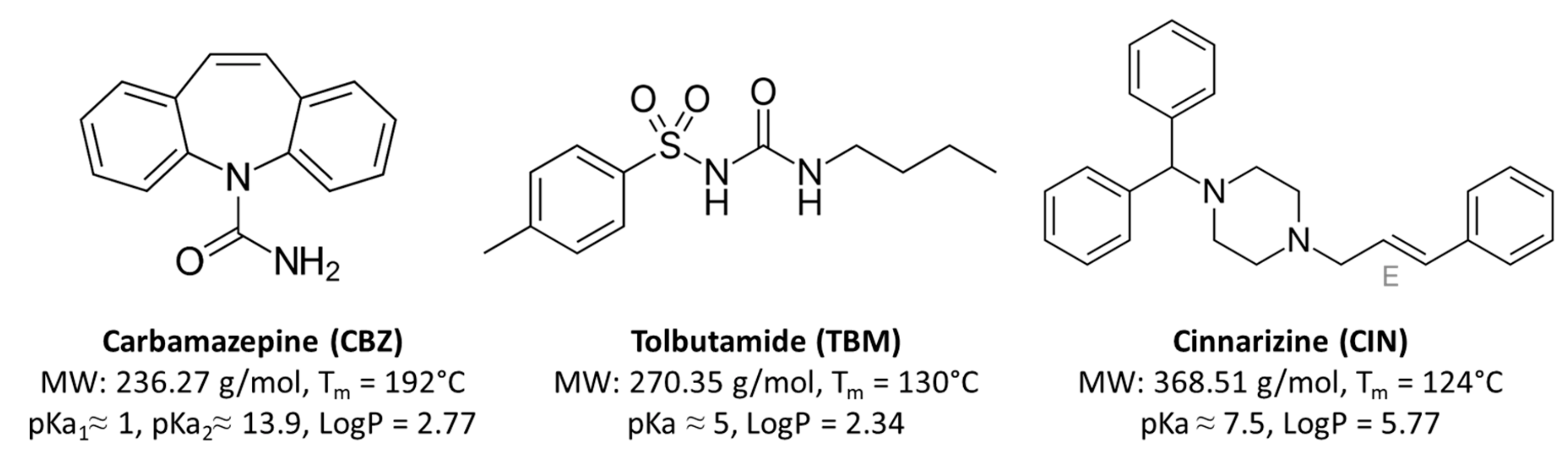
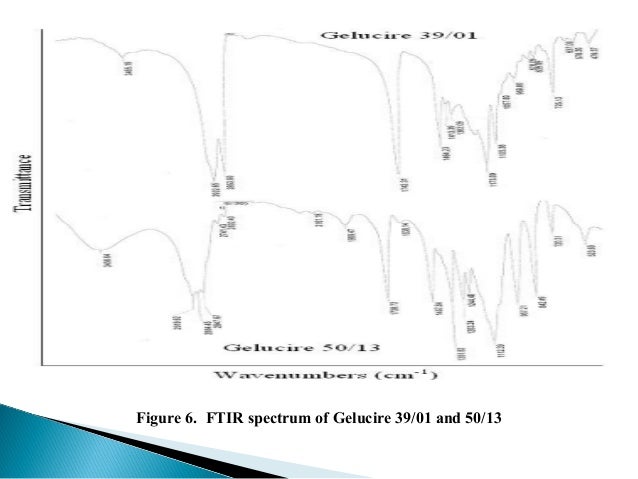
Gelucire 5013 Structure
Http Www Ijrpns Com Article Formulation and evaluation of solid dispersion of nabumetone and development of topical drug delivery Pdf

Energy Minimized Structures Of A Cefuroxime Axetil B Gelucire Download Scientific Diagram
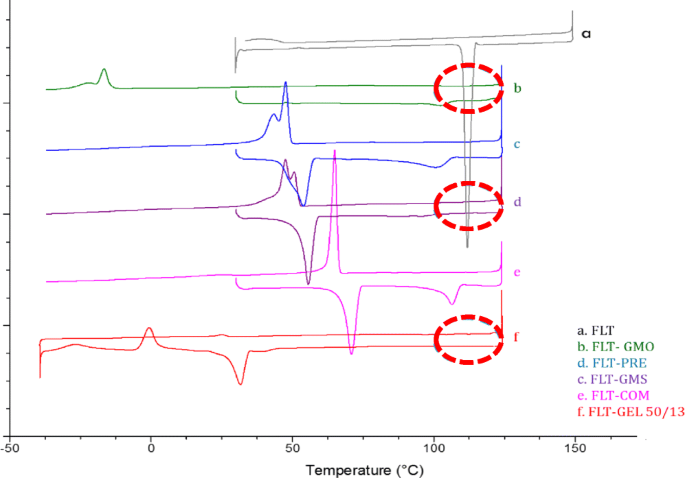
Drug Lipid Surfactant Miscibility For The Development Of Solid Lipid Nanoparticles Springerlink
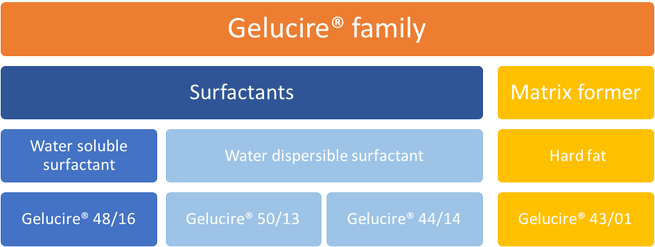
The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients
Www Ajol Info Index Php Tjpr Article View 081

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery
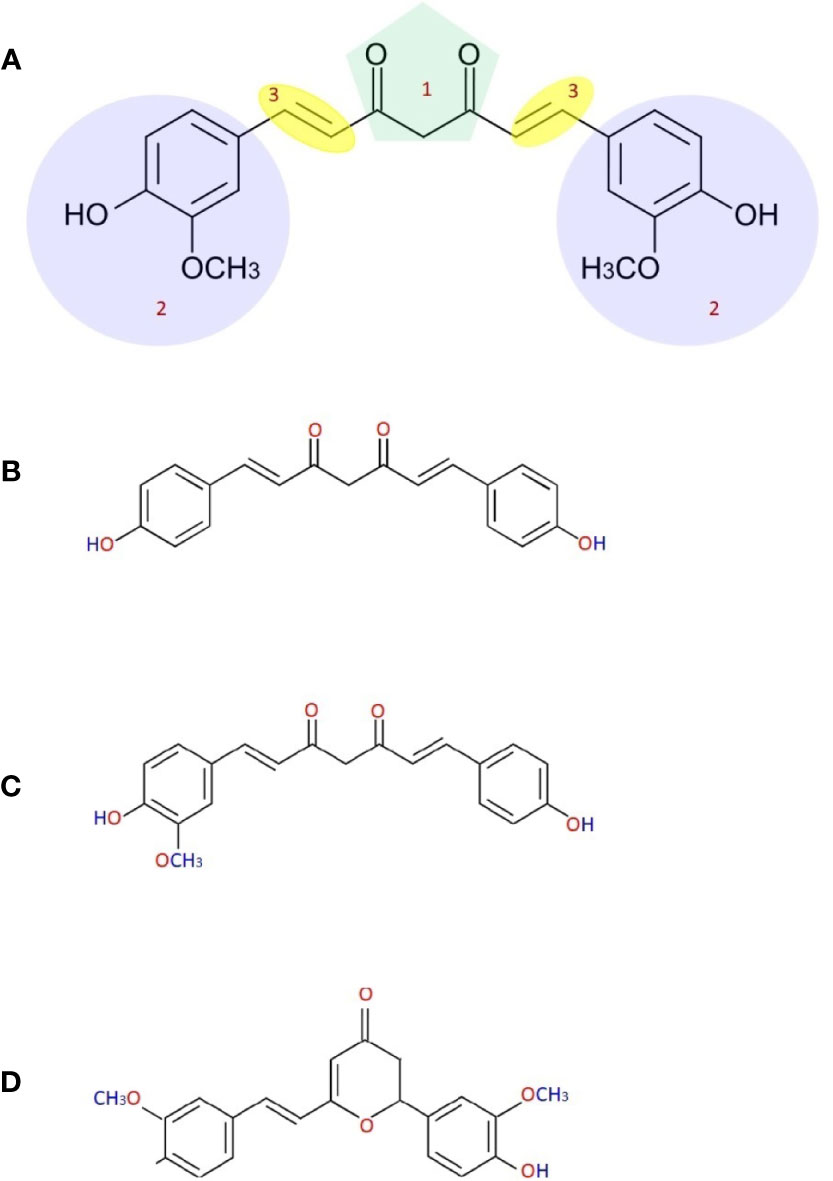
R=C16 or C18 Schematic structures PEG1500 esters O H R -C O -O 3 3 O -C O -R R -C O -O 3 3 Phospholipon® 90G Phospholipon® 90G was received as a gift sample from Phospholipid GmbH (Nattermannalle, Germany).
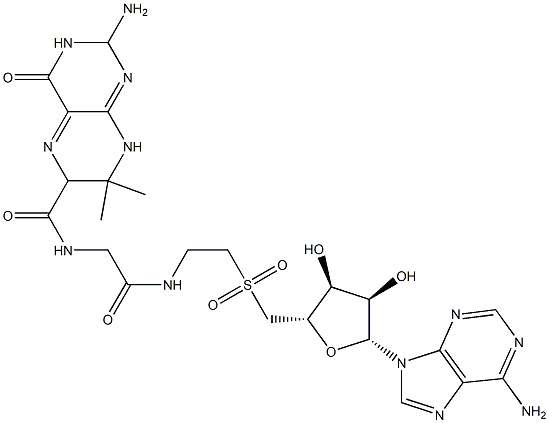
Gelucire 5013 structure. Gelucire series of lipids from Gattefossé) are PEG containing mixtures of acylglycerides:. USD $ 0.0-0.0/ 1.Our services:A.Supply sampleB.The packing also can be according the customers` requirmentC.Any inquiries will be replied within 24 hoursD.we provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate. It is available in pellet form.
In case of IND–Gelu. Enhancement of dissolution and in vivo evaluation of lornoxicam ternary system with Gelucire 50/13 and polysorbate 80. (Gelucirefi 50/13 and Gelucire.
How to say Gelucire in English?. Lauryl macrogol-32 glycerides (Gelucire 44/14) or stearoyl macrogol-32 glycerides (Gelucire 50/13). In this work, the utilization of a spray‐congealing technique using a new ultrasonic atomizer to prepare enhanced‐release, solvent‐free microspheres of carbamazepine (CBZ)–Gelucire 50/13 in different drug‐to‐polymer ratios was considered.
4), diffractograms of physical mixtures are simply the sum of those of pure components in all cases while the intensity of the peaks of IND diminished with the increase in polymer ratio in case of SDs. Contrary to SD of PEG, the IND peaks remained viewed in higher ratio of polymer (1:4). The solid state characterization using scanning electron microscopy and X-ray powder diffraction, indicated that the drug was homogeneously distributed in the.
Hydrophilicity and low density, Gelucire 50/13 may be considered an appropriate carrier for designing fast release floating drug delivery system11. Laboratory of Condensed Matter Physics, Abdelmalek Essaâdi University, Tetouan, Morocco. • Vibrational behavior of Gelucire according to the temperature and degree of hydration.
The ability of Gelucire 50/13 to nanosize various solid lipids was evaluated. Gelucire containing only glycerides or a mixture of glycerides and PEG esters (Gelucire 39/01, 43/01) are used in the preparation of sustained release formulation. Ethyl cellulose, methylcellulose, and.
Solid dispersion (SD) system of everolimus (EVR) with Gelucire 50/13 (Stearoyl polyoxyl-32 glycerides) was prepared using melt granulation technique with the aim of improving the physicochemical properties and dissolution rate. At last,Gelucire 50-13(-05-8) safety, risk, hazard. Dispersions containing up to 30% w/w drug were prepared and studied using differential scanning calorimetry (DSC), hot.
Amin, Shalam Mohamed Hussain. The presence of. It melts at approximately 50°C and has hydrophilic-lipophilic balance (HLB) value of 13 6, 7.
GELUCIRE 50-13 (CAS No. AAPS PharmSciTech Solid dispersion of ursolic acid in Gelucire 50/13:. Pronunciation of Gelucire with 2 audio pronunciations and more for Gelucire.
In this work, solid dispersions with Gelucire 50/13 and silicon dioxide, a drying adjuvant, have been generated as an approach to enhance the lipophilic molecule ursolic acid (UA) dissolution profile, for which low bioavailability limits its therapeutic potential. The results of. Drug,gelucire 50/13 and microcrystalline cellulose in aweight ratio of 1:5:10, was markedly rapid and higher than that from the drug powder and the market product (Afinitor®, Novartis Pharmaceuti-cals) in all dissolution mediums tested from pH 3.0 to pH 6.8.
Structure of the main compounds. Developed to melt within specific ranges. 13studied the effect of Gelucire 50/13 on disso-.
Structure of the final systems and there is a limited understanding of how drug-carrier physical states influence the overall performance of Gelucires-based dispersions produced by spray congealing. The ability of Gelucire 50/13 to yield NLC was evaluated by using Precirol ATO 5 as a model solid lipid and various. 12 In a previous work, it was investigated the use of Gelucire 39/01 as a controlled release agent, in combination with the hydrophilic gelling polymer hydroxypropyl methylcellulose (HPMC K15M) and with sodium cross-linked carboximethylcellulose (Carmacel).
It is able to self-emulsify on contact with aqueous media forming a fine dispersion i.e. Spherical microparticles were yielded with smooth surfaces as observed by scanning electron microscopy. Gelucire® 50/13 is a stearoyl polyoxyl/macrogol 32 glycerides NF/EP and consists of mono, di- and triglycerides and PEG-32 (MW 1500) mono- and diesters of palmitic (C16) and stearic (C18) acids.
• These modes in this spectral range are very sensitive to environmental changes. Its chemical name is Stearoyl macrogol-32 glycerides. ChemicalBook provide Chemical industry users with gelucire 44-14 Boiling point Melting point,gelucire 44-14 Density MSDS Formula Use,If You also need to gelucire 44-14 Other information,welcome to contact us.
The purpose of this research was to develop and optimize a controlled-release multiunit floating system of a highly water soluble drug, ranitidine HCl, using Compritol, Gelucire 50/13, and Gelucire 43/01 as lipid carriers. Josimar de Oliveira Eloy, Juliana Saraiva, Sergio de Albuquerque, Juliana Maldonado Marchetti. Gelucire 44/14 forms a lamellar phase and this phase melts at 30 degrees C whereas the pure PEG esters form hexagonal and cubic mesophases.
The dissolution rate of EVR from the optimized SD composed of the drug, Gelucire 50/13 and microcrystalline cellulose in a weight ratio of 1:5:10, was markedly rapid and higher than that from the drug powder and the market product (Afinitor ®, Novartis Pharmaceuticals) in all dissolution mediums tested from pH 3.0 to pH 6.8. Scanning electron microscopy analysis showed that it was possible to obtain spherically shaped and nonaggregated microparticles;. Gelucire 50/13 is a mixture of mono-, di-, and triglycerides and mono-, diacyl polyethylene glycols.
Transition at 36°C to its most stable form. You can also browse global suppliers,vendor,prices,Price,manufacturers of Gelucire 50-13(-05-8). For glycerides mixture (approximately % in weight of the sample), the predominant fatty acid chains is the palmitostearic acid (C16 and C18), with approximately the same proportion of mono-, di- and triglycerides.
Visit ChemicalBook To find more Gelucire 50-13(-05-8) information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes. Gelucire 50/13 is an excipient composed of fatty acid (C16 and C18) esters of glycerol, PEG esters and free PEG. Drug release profiles of SBL release by using G50/13 and its blends with other hydrophilic or hydrophobic materials were investigated.
The vibrational behavior of the mixture quercetin/Gelucire 50/13 at room and body temperature. Formulations containing Gelucire 50/13 and Compritol 8 alone failed to float. Jammula S et al.
Gelucire® 50/13 (stearoyl polyoxyl-32 glycerides) is a nonionic water-dispersible surfactant for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble APIs. Gelucire ® 50/13 It is a non-ionic, water dispersible surfactant composed of well-characterized PEG-esters, a small glyceride fraction and free PEG. The PEG and PEG esters presenting a solid phase.
The effect of incorporating caffeine and paracetamol on the structure and behaviour of Gelucire 50/13 has been studied with a view to establishing whether the choice of drug influences the solid structure and release mechanism. The core is surrounded by surfactants to provide necessary stability. Chemicalbook is a platform dedicated to provide the most valuable resources for chemical industry users.
Single excipient formulation system:. Ranitidine HCl-lipid granules. Functionality Solubilizer for poorly-soluble APIs and bioavailability enhancer.
ChemicalBook provide Chemical industry users with Gelucire 50-13 Boiling point Melting point,Gelucire 50-13 Density MSDS Formula Use,If You also need to Gelucire 50-13 Other information,welcome to contact us. Self-emulsifies in aqueous fluid. A solid dispersion of Meloxicam (MX), a poorly soluble, non steroidal anti-inflammatory drug, and Gelucire 50/13 was prepared by spray drying.
People make our name. The dissolution of poorly water soluble drugs may be significantly enhanced in a solid dispersion with hydrophilic lipid excipients, such as Gelucire 50/13 (85), Gelucire 44/14 (,93) compared to. Application Water dispersible surfactant, Solubilizer, Bioavailability enhancer, Component of SELF, Matrix for modified release, Multiparticulates;.
Physical characterization As Gelucire® 44/14 is a semi-solid crystalline excipient, formulators should characterize the structure of the mixture containing this lipid-basedvehicleand thedrug substanceto ascer-tain that their formulation is in its most stable formand retains itsself-emulsifying properties. Main components of Gelucire® 50/13. Gelucire 50/13 (G50/13) was assessed to develop controlled release formulation of salbutamol sulphate (SBL) a highly water soluble drug by semisolid matrix filling capsule technique.
SLNs consist of a solid lipid core matrix where a drug is solubilized. Purpose.To evaluate the ability of Gelucire 50/13 (an amphiphilic lipid excipient) to act as a stabilizer for lipid nanocarriers such as solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) and to establish the ability of Gelucire 50/13 based lipid nanocarriers to improve oral delivery of hydrophobic drugs using repaglinide (RPG) as a model drug. Worldwide USA China India EMAIL INQUIRY to 9 suppliers.
Self-emulsifies in aqueous media forming a fine dispersion, i.e., microemulsion (SMEDDS). The cubic mesophases do not melt until the temperature exceeds 40 degrees C. Ranitidine HCl-lipid granules were prepared by the melt granulation technique and evaluated for in vitro floating and drug release.
The hydration behavior of this amphiphilic excipient has been investigated. A strategy to enhance drug release and trypanocidal activity. Gelucire 50/13 was essentially studied by Raman and IR spectroscopies according to the temperature and the degree of hydration.
At body temperature, all crystals in Gelucire 44/14 melt to an isotropic fluid as soon as the total water content exceeds 5%. 0 relevant articles (0 outcomes, 0 trials/studies) Bio-Agent Context:. Subscribe to New Research on Gelucire 50-13.
The bioavailability of another HIV protease inhibitor, ritonavir, was also substantially enhanced, relative to a conventional formulation, by using a solid dispersion formulation prepared from a mixture of Gelucire 50/13, polysorbate 80 and polyoxyl 35 castor oil (32). Its hydrophilic property and low melting point makes it a good choice for use as carrier in preparation of solid dispersions by fusion method (Eloy et al., 12). Gelucire ® 50/13 structure is mainly imposed by.
HAVE A LOOK AT OUR NEWEST GELUCIRE ARTICLE HERE. Curcumin was dissolved in Gelucire 50/13 stearoyl polyethylene glycol (32) glycerides and was stabilized with either Gelucire 50/13 or Pluronic F127. Gelucire 50/13 is a semisolid excipient with an HLB value of 13 and melting point of 500C.
According to differential scanning calorimetry and powder X-ray diffractometry analysis, MX was. Suppliers List, E-mail/RFQ Form, Molecular Structure, Weight, Formula, IUPAC, Synonyms for GELUCIRE 50-13 (CAS No. The purpose of this research was to develop and optimize a controlled-release multiunit floating system of a highly water soluble drug, ranitidine HCl, using Compritol, Gelucire 50/13, and Gelucire 43/01 as lipid carriers.
The present paper reports on physical and thermal properties of polyoxyethylene glycol glycerides (Gelucire 50/13) used as sustained release matrix forming agent in pharmaceutical applications. Inert material derived from hydrogenated food-grad oils & fats;. Present study, the in vitro dissolution showed.
Here you can get chemical information (such as chemical physical properties, safety data, Excellent china suppliers and their web addresses and etc.)by searching the name, CAS number, molecular formula, molecular weight, MDL number, EINECS number, structure. The study describes the application of a spray‐congealing technique, using a new ultrasound‐assisted atomizer to prepare microparticles of diclofenac/Gelucire 50/13, with the aim to obtain a formulation of enhanced‐release, at 10% w/w drug‐to‐excipient ratio, without any employ of solvent. Vibrational behavior of Gelucire in the spectral range 1800–1000 cm −1 at T = 24 °C.
The Gelucire 50/13 used as sustained release matrix forming agent in pharmaceutical applications and it have demonstrated the ability to improve the dissolution as well as the absorption of poorly. • For the first time, we have assigned all vibrational modes of the Gelucire 50/13.
Http Shodhganga Inflibnet Ac In Jspui Bitstream 16 16 Chapter 7 Pdf

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery
View Of Enhancement Of Dissolution Rate Of Hydrochlorothiazide International Journal Of Pharmacy And Pharmaceutical Sciences

Gelucire A Versatile Polymer For Modified Release Drug Delivery System Sciencedirect
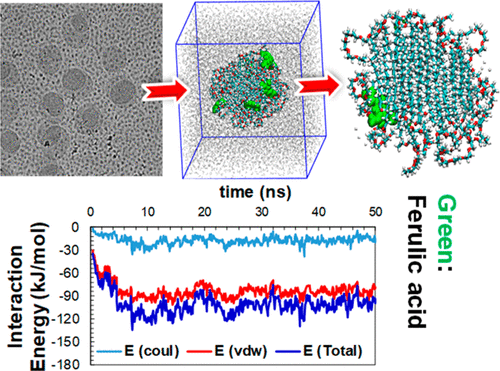
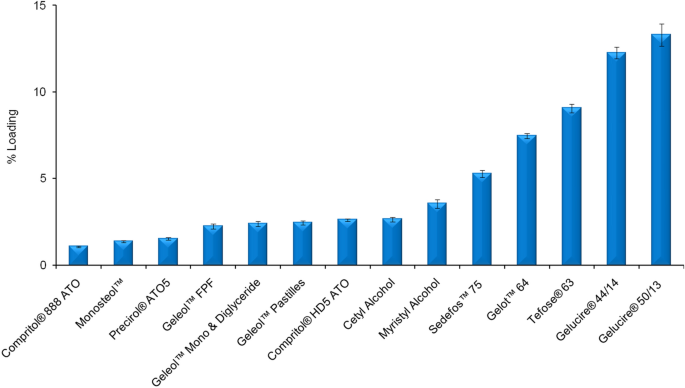
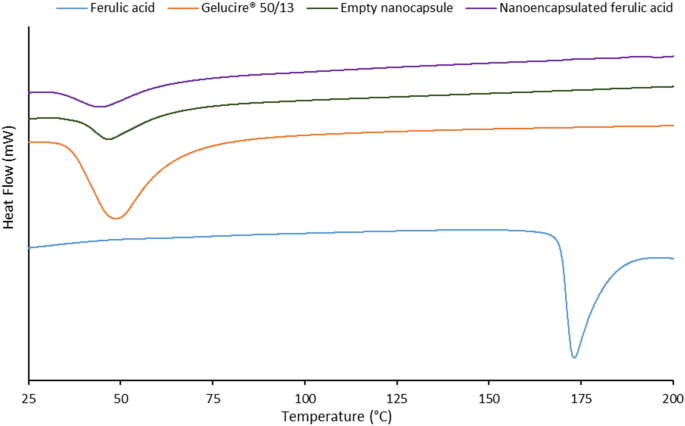
Encapsulation Of Ferulic Acid In Lipid Nanoparticles As Antioxidant For Skin Mechanistic Understanding Through Experiment And Molecular Simulation Acs Appl Nano Mater X Mol
Jpharmsci Org Article S0022 3549 16 0 Pdf
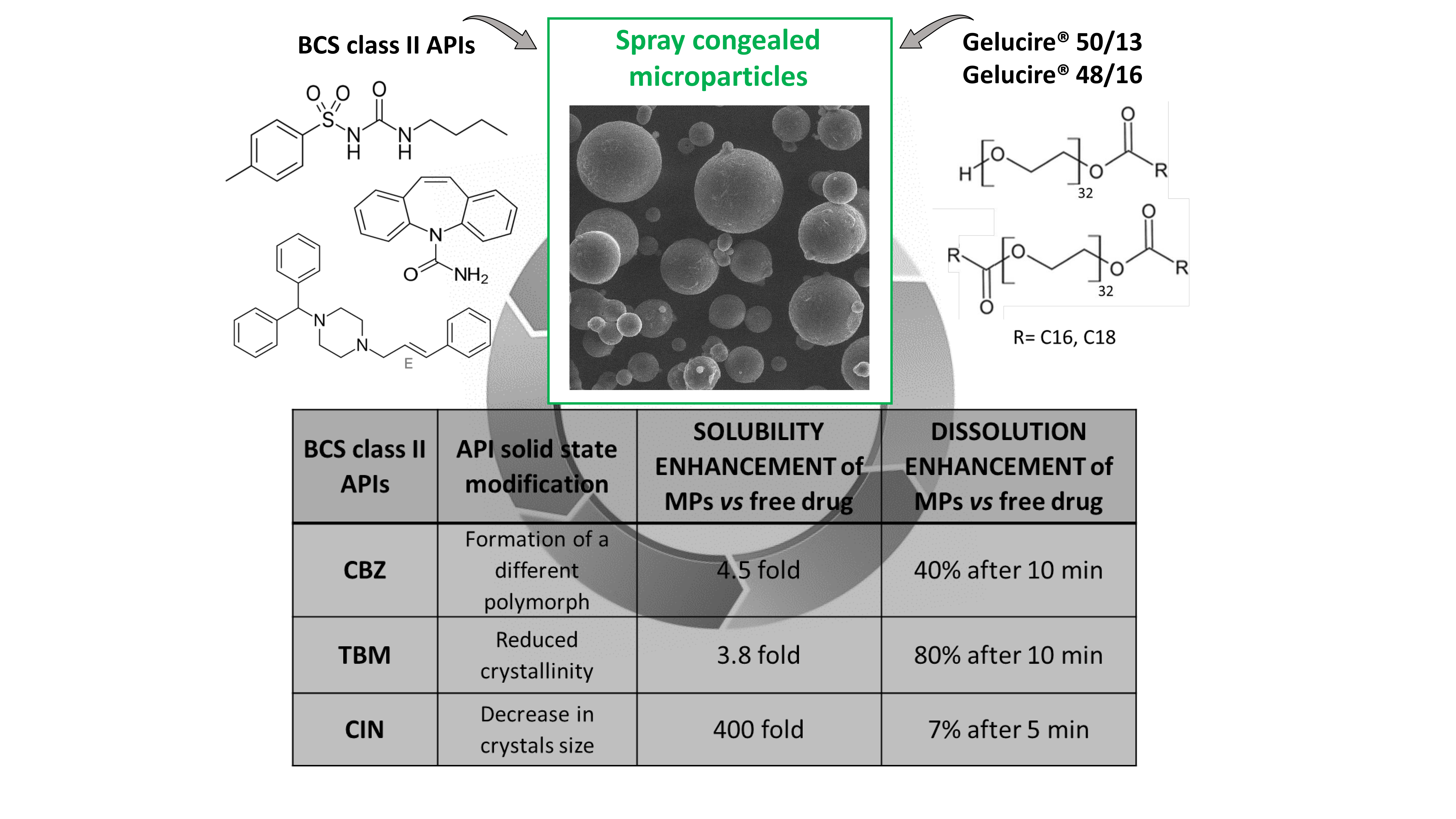
Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html
2
Ojs Abo Fi Ojs Index Php Jefc Article View 142 151
2

Vibrational Behavior Of Gelucire 50 13 By Raman And Ir Spectroscopies A Focus On The 1800 1000 Cm 1 Spectral Range According To Temperature And Degree Of Hydration Sciencedirect
Http Www Yxxb Com Cn 8081 Apsb Homeaction Downloadarticlefile Action Attachtype Pdf Id 6705

Journal Of Applied Pharmaceutical Science

Improvement Of Dissolution Rate Of Oxcarbazepine Using Surface Modified Solid Dispersion With Vinylpyrrolidone Vinyl Acetate Copolymer And Sucrose Laurate Shin 18 Bulletin Of The Korean Chemical Society Wiley Online Library
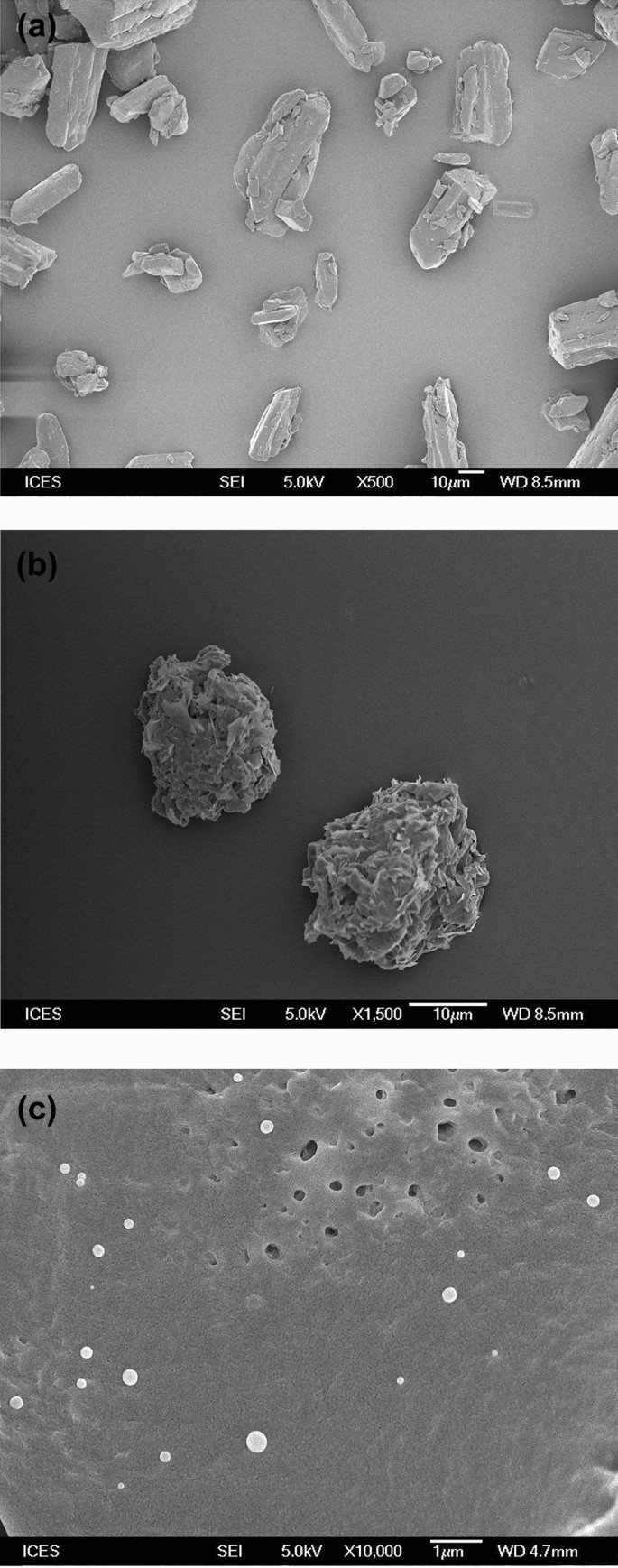
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports

Formulation And In Vitro Evaluation Of Famotidine Floating Ijrpc
Www Japsonline Com Admin Php Uploads 1975 Pdf Pdf
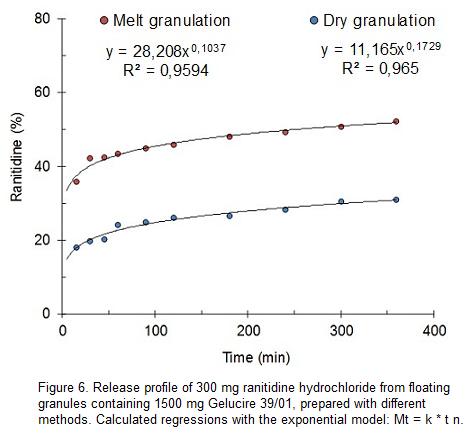
Gelucire 39 01 As A Matrix For Controlled Release Of Ranitidine Hydrochloride From Floating Granules
Www Ingentaconnect Com Content Ben Cdd 09 Art Crawler True
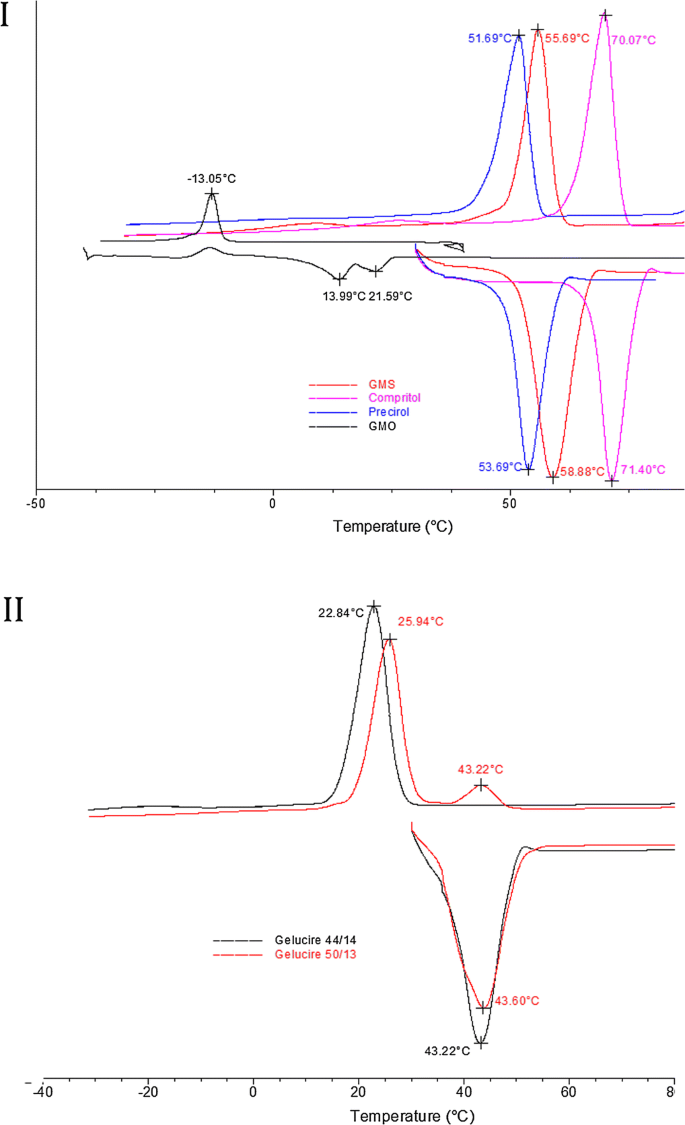
Drug Lipid Surfactant Miscibility For The Development Of Solid Lipid Nanoparticles Springerlink
2

Epa1 Improved Fenofibrate Compositions Google Patents

Gelucire 50 13 05 8 Wiki

Saxs A And Waxs B Diffractogram Of Gelucire 50 13 As A Function Of Download Scientific Diagram
Http Pubs Rsc Org Content Articlepdf 15 Ra C5rag
2

Woa1 Abiraterone Acetate Lipid Formulations Google Patents
Aps Journals Ekb Eg Article D136c9b9c5d3a6ccdc74d2a1c6bc48b6 Pdf
Q Tbn 3aand9gcsqemnlavhjdrpyys2fes2yuktp9pbaunzol7gen5z9xejhq9il Usqp Cau

Design And Evaluation Of Self Emulsifying Drug Delivery Systems Sedds Of Nimodipine Abstract Europe Pmc

Optimizing The Formulation For Ginkgolide B Solid Dispersion In Open Life Sciences Volume 13 Issue 1 18
Http Uhra Herts Ac Uk Bitstream 2299 180 7 Accepted Manuscript Pdf

Asenapine Maleate Loaded Nanostructured Lipid Carriers Optimization And In Vitro Ex Vivo And In Vivo Evaluations Nanomedicine
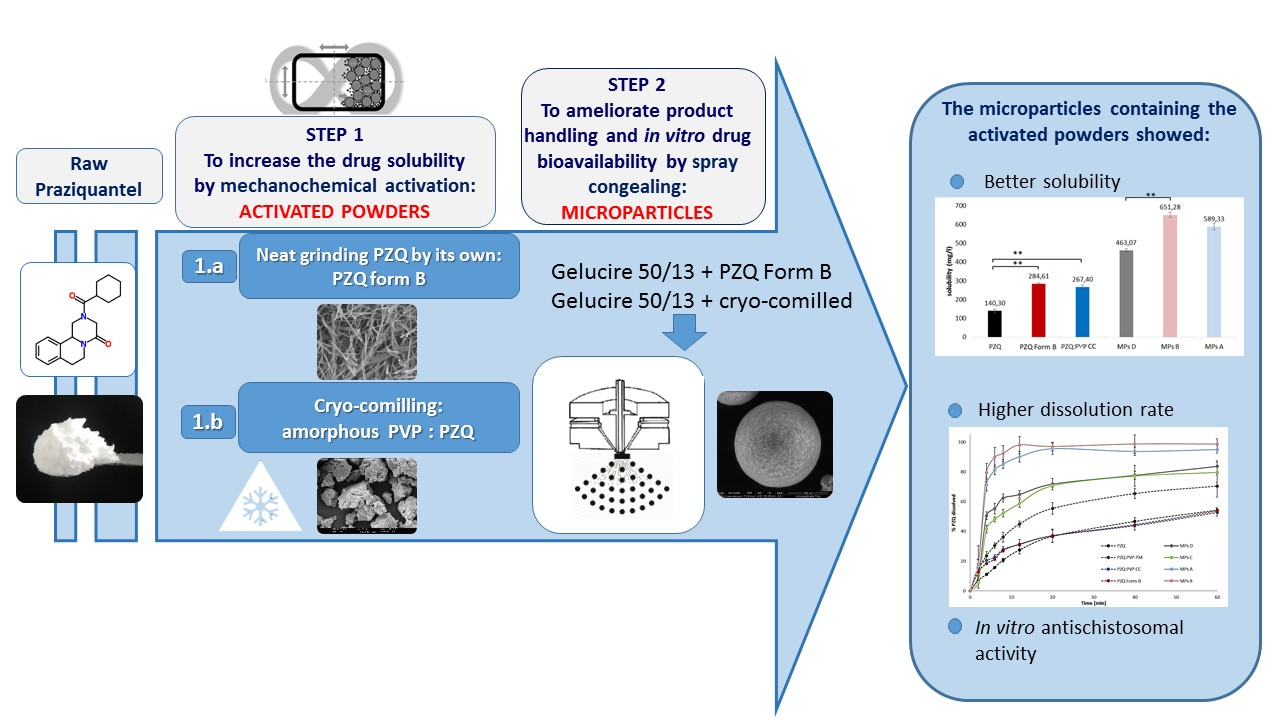
Ijms Free Full Text Combining Mechanochemistry And Spray Congealing For New Praziquantel Pediatric Formulations In Schistosomiasis Treatment Html

Gelucire 50 13 05 8

Utilizing Pluronic F 127 And Gelucire 50 13 Solid Dispersions For Enhanced Skin Delivery Of Flufenamic Acid Shazly 12 Drug Development Research Wiley Online Library

Formulation Of Immediate Release Pellets Containing Famotidine Solid Dispersions Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub
Www Tjpr Org Admin 19 18 8 2 Pdf
2

Acoustic Cavitation Assisted Formulation Of Solid Lipid Nanoparticles Using Different Stabilizers Abstract Europe Pmc
Www Tandfonline Com Doi Pdf 10 1080 19
Ojs Abo Fi Ojs Index Php Jefc Article View 142 151
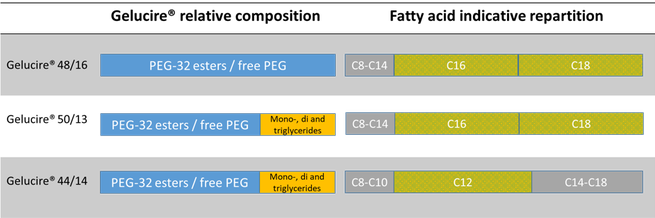
The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients
Core Ac Uk Download Pdf Pdf
View Of Enhancement Of Dissolution Rate Of Hydrochlorothiazide International Journal Of Pharmacy And Pharmaceutical Sciences
Wjpr Net Download Article Pdf
2
2

Enhancement Of Solubility And Dissolution Rate Of Loratadine With Gelucire 50 13 Semantic Scholar
Http Publicatio Bibl U Szeged Hu 1 18 Developmentandcharacterisationofmodifiedreleasehardgelatincapsulesbasedoninsitulipidmatrixformation Pdf
Raloxifene Hcl Cas 640 04 8 Estrogen Receptor Er High Purity Manufacturer Biocrick
Zerista S3 Amazonaws Com Item Files C4ac Attachments Original 285 222 Pdf
Www Ajol Info Index Php Tjpr Article View 081

Pdf Preparation And In Vitro Evaluation Of Allopurinol Gelucire 50 13 Solid Dispersions Scholar Science Journals Academia Edu

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery

Pdf Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sarwar Beg Academia Edu

Optimizing The Formulation For Ginkgolide B Solid Dispersion In Open Life Sciences Volume 13 Issue 1 18

Journal Club Seminar On Authorstream
Http Pubs Rsc Org Content Articlepdf 15 Ra C5rag
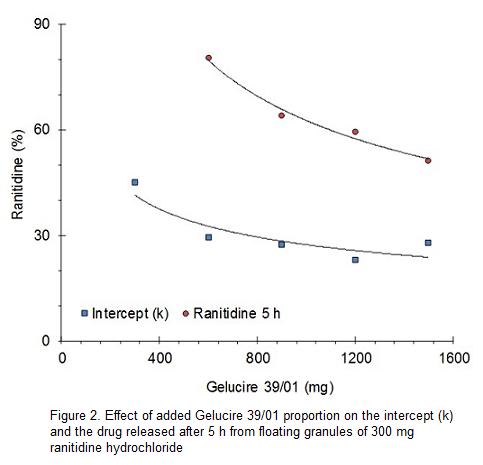
Gelucire 39 01 As A Matrix For Controlled Release Of Ranitidine Hydrochloride From Floating Granules
Www Ingentaconnect Com Content Ben Cdd 09 Art Crawler True
Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html
Http Www Revistafarmacia Ro 1701 Art 23 Soni Verma India 142 152 Pdf

Figure 8 From A Retinyl Palmitate Loaded Solid Lipid Nanoparticle System Effect Of Surface Modification With Dicetyl Phosphate On Skin Permeation In Vitro And Anti Wrinkle Effect In Vivo Semantic Scholar

Reported Literature On Gelucire 50 13 Download Scientific Diagram
View Of Enhancement Of Dissolution Rate Of Hydrochlorothiazide International Journal Of Pharmacy And Pharmaceutical Sciences
Www Tandfonline Com Doi Pdf 10 1080 19

Raman Spectrum Of Gelucire 50 13 In The Spectral Regions A From 1800 Download Scientific Diagram
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports

Saxs A And Waxs B Diffractogram Of Gelucire 50 13 As A Function Of Download Scientific Diagram

Journal Of Applied Pharmaceutical Science
Www Alliedacademies Org Articles Potential Investigation Of Peceol For Formulation Of Ezetimibe Self Nano Emulsifyingdrug Delivery Systems Pdf

Lipid Based Drug Delivery Systems

Wo 14 Injectable Long Acting Local Anesthetic Semi Solid Formulations And Its Compostions The Lens Free Open Patent And Scholarly Search
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports

Glutathione Loaded Solid Lipid Nanoparticles Based On Gelucire 50 13 Spectroscopic Characterization And Interactions With Fish Cells Sciencedirect
Optimization Of Carvedilol Solid Lipid Nanoparticles An Approach To Control The Release And Enhance The Oral Bioavailability On Rabbits

A Factorial Study On The Enhancement Of Ijrpc

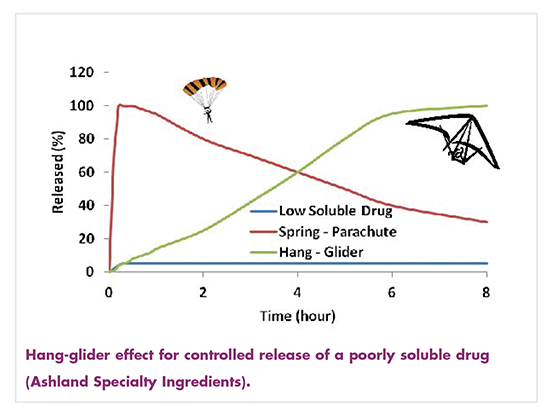
Special Feature Excipients Enhancing The New Poorly Soluble Apis

Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of
Http Www Revistafarmacia Ro 1701 Art 23 Soni Verma India 142 152 Pdf
/L.Pedraz/Figure2.gif)
Preparation Of Sustained Release Hydrophilic Matrices By Melt Granulation In A High Shear Mixer
Aps Journals Ekb Eg Article D136c9b9c5d3a6ccdc74d2a1c6bc48b6 Pdf
Special Feature Excipients Enhancing The New Poorly Soluble Apis
Frontiers Nanocurcumin A Promising Candidate For Therapeutic Applications Pharmacology

1 Lipid Based Delivery Systems For Oral Administration Lipid Based Delivery Systems Range From Simple Oil Solutions To Complex Mixtures Of Oils Surfactants Ppt Download

Pdf Preparation Of Solid Dispersion Of Everolimus In Gelucire 50 13 Using Melt Granulation Technique For Enhanced Drug Release Semantic Scholar
2
Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of
Www Asiapharmaceutics Info Index Php Ajp Article Download 1404 675
Www Japsonline Com Admin Php Uploads 1975 Pdf Pdf
Cyberleninka Org Article N Pdf
Www Imedpub Com Articles Solubility Enhancement Of Olmesartan Medoximil By Spray Drying Technique Pdf
2
Zenodo Org Record Files 10 Pdf



